|
D-TARTARIC ACID | ||
|
PRODUCT IDENTIFICATION |
||
|
CAS NO. |
147-71-7 |
|
| EINECS NO. |
205-695-6 | |
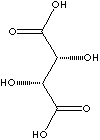
| FORMULA | HOOCCH(OH)CH(OH)COOH | |
| MOL WT. | 150.09 | |
|
H.S. CODE |
2918.12 | |
| SMILES |
|
|
|
TOXICITY |
||
| SYNONYMS | [S-(R*,R*)]-2,3-dihydroxy-Butanedioic acid; | |
| (-)-(2S,3S)-Tartaric Acid; (2S,3S)-(-)-Tartaric Acid; D-(-)-Dihydroxysuccinic acid; D-(-)-Tartaric Acid; L-Tartrate; (S,S)-Tartaric acid; (S,S)-Tartrate; 2,3-Dihydroxysuccinic acid; | ||
|
CLASSIFICATION |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
white crystalline powder | |
| MELTING POINT | 166 - 172 C | |
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER |
soluble | |
| pH | ||
| VAPOR DENSITY |
| |
|
AUTOIGNITION |
||
|
NFPA RATINGS |
Health: 2 Flammability: 1 Reactivity: 0 | |
|
REFRACTIVE INDEX |
||
| FLASH POINT |
| |
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
| Tartaric Acid, also called dihydroxysuccinic acid [HOOC(CHOH)2COOH]), is a white crystalline naturally occurring carboxylic acid; melting at 171 C, soluble in water and alcohols. It is obtained natually as a by-products of wine fermentation along with its salts. This natural acid is used as an antioxidant in food. Tartaric acid has two asymmetrical carbon atoms and three chiral isomers; the dextro-, levo-, (optically active) and meso- forms (optically inactive). The d- and l-tartaric acids are said to be enantiomorphs (each molecule is asymmetrical and has the mirror image of the other). There are two asymmetrical carbon atoms in meso-tartaric acid, but the molecule is symmetrical and does not exhibit optical activity; the optical activity is internally compensated, the effect of one asymmetrical carbon atom balancing the effect of the other. A pair of optical isomers such as d-tartaric acid and meso-tartaric acid, which are not enantiomorphs, are called diastereoisomers. Tartaric Acid is a useful raw material for the synthesis of other chiral compounds. L-tartaric acid (called also d-2,3-dihydroxysuccinic acid or l-2,3-dihydroxybutanedioic acid) is chiefly found in many plant especially grape. This form can be partially converted to the others by heating it with an aqueous alkali (potassium hydroxide) as the isomeric forms differ from each other in boiling points. It can be synthesized by the reaction of maleic acids or fumaric acids with aqueous potassium permanganate. Tartaric acid is biodegradable and no pollution problems are known. Tartaric acid is used chiefly in the form of its salts, e.g., cream of tartar (potassium hydrogen tartrate), Rochelle salt (potassium sodium tartrate) and Tartar Emetic (antimony potassium tartrate). It is used to enhance flavours in foods, confectionery and beverages. It is used as a chemical intermediate and a sequestrant and in tanning, ceramics, photography, textile processing, mirror silvering, and metal coloring. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white crystalline powder |
|
|
PURITY |
99.0% min |
|
| OPTICAL ROTATION |
-12° ~ -14° ((c=10 in water) |
|
| SULPHATED ASH |
0.1% max |
|
| IRON |
2ppm max |
|
|
HEAVY METALS |
10ppm max |
|
| TRANSPORTATION | ||
| PACKING | 25kgs in bag | |
| HAZARD CLASS | Not regulated | |
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 26-36 | ||